Cytogenetics and Molecular Genetics
Chemical Pathology
Notes
- The Department of Chemical Pathology provides a postal service for samples needing to be sent to the South West Genomic Laboratory Hub, as outlined in the the 2021/2022 National Genomic Test Directory
- Blood samples must be accompanied by an appropriate request form
- Results will be returned directly to the requesting clinician.
In October 2021, a new version of the national genomic testing eligibility criteria was published by NHS England. This contains all of the requirements that must be met in order for genetic testing to be nationally funded, and will ensure that there is standardisation of genetic requesting across the country. This means that samples that are sent to the genomic hub for processing on patients who do not meet the criteria for testing for the conditions being investigated are unlikely to be processed. Therefore, to avoid samples being taken and sent unnecessarily, requestors should ensure they have checked this document prior to samples being sent. The document and further information from NHS England can be found here
Please note that any tissue samples following miscarriage or termination of pregnancy must be sent to the Antenatal Clinic and are not dealt with by Chemical Pathology.
Sample requirements
Cytogenetics requires lithium heparin whole blood; molecular genetics requires EDTA whole blood.
For cytogenetics in adults, blood taken into a 6mL lithium heparin tube

For cytogenetics in children, blood taken into a 2mL lithium heparin tube
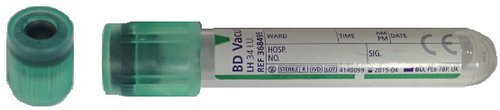
For cytogenetics in neonates, blood taken into a 0.8mL minicollect lithium heparin tube
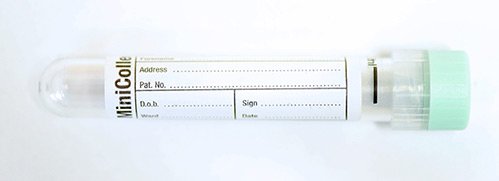
For molecular genetics in adults, blood taken into an 4 mL EDTA tube
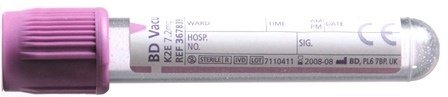
For molecular genetics in children, blood taken into an 2 mL EDTA tube
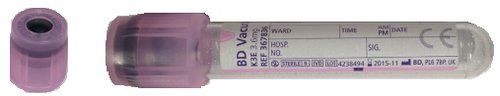
For molecular genetics in neonates, blood taken into a 1 mL EDTA tube
Storage/transport
Send at ambient temperature to the laboratory. If unavoidable, samples can be stored refrigerated overnight.
Required information
The 2021/2022 National Genomic Test Directory specifies which genomic tests are commissioned by the NHS in England, the technology by which they are available, and the patients who will be eligible to access to a test. For more information on eligibility criteria and testing directory, please visit NHS England
Relevant clinical details including details of consent where appropriate should be detailed on the request form. Please also include test codes as listed in the National Genomic Directory, where possible.
Turnaround times
Samples received in Chemical Pathology are transported to Bristol on the next available transport run (once daily, Monday to Friday only - currently approximately 13:30)
Results will be sent direct to the requesting clinician within 4-6 weeks.
Further information
To learn more visit either South West Genomic Laboratory Hub website, or Bristol Genetics website
Page last updated 10/12/2024