Peripheral intravenous (IV) cannula
This page gives information to patients having a peripheral intravenous (IV) cannula tube inserted into a vein. This is usually into the back of your hand or your arm (see Figure 1 and 2).
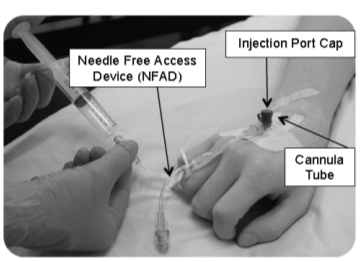
The procedure to insert the cannula tube will be carried out by a member of the healthcare team trained to perform cannulation.
A member of staff will explain the procedure to you and answer any questions you may have. You will then be asked for your consent to go ahead with the procedure.
Gloucestershire Hospitals NHS Foundation Trust provide training placements for medical, nursing and other health professions. A student may be present when your cannula tube is inserted or they may carry out your cannulation under supervision. If you do not wish students to be present or insert your cannula, please let the member of staff looking after you know.
What is a cannula?
A cannula is a small, soft plastic tube that is placed into a vein, usually in your hand or arm. It’s inserted using a fine, safety needle. Once the cannula is in the vein, the needle is removed leaving just the flexible tube in place. The cannula has one or more connectors which allow staff to give fluids and medications directly into your bloodstream. Sometimes a cannula may be inserted into another part of your body such as your foot or leg.
Why do I need a cannula?
You have been prescribed medication, fluids or blood products to be given intravenously (directly into your bloodstream) for either:
- short term treatment such as diagnostic tests, where a dye or other substance needs to be injected or you require an anaesthetic.
- long term treatment where you may require several days of intravenous medication such as antibiotics for an infection.
Your treatment may be given as an infusion (drip) or in a syringe (bolus) usually through a Needle Free Access Device (NFAD) (see Figure 1).
Cannula preparation
Your veins will be assessed to find the best site for insertion. A tourniquet (tight band) may be applied to make your veins more visible. If needed, local anaesthetic (cream or injection) will be discussed. Hair at the insertion site may be trimmed with sterile scissors.
How will the cannula be inserted?
Once the cannula tube has been inserted, the tourniquet and safety needle will be removed. The needle will be placed immediately into a sharp’s container.
The cannula tube will remain in your vein to provide access for your treatment.
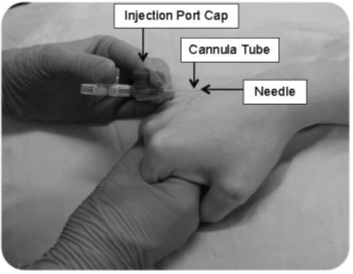
You may feel a brief pain or discomfort as the safety needle is inserted. Once the safety needle has been removed the pain will go. If you feel pain during or after the procedure, please tell the member of staff looking after you.
How the staff will take care of your cannula
The member of staff who inserts the cannula or gives your treatment will take care to help prevent any infection by following an aseptic procedure. They will also inspect the site at least twice a day to check that it is healthy and can still be used.
Before cannulation staff will:
- clean their hands.
- wear an apron and gloves.
- clean your skin with a disinfectant wipe and allow to air dry.
- apply a sterile dressing to keep the cannula in place and add a date and time sticker to the edge of your dressing.
- check the cannula is working by flushing it with saline fluid after insertion. This fluid may feel cool when it goes in but should not be painful.
At every access staff will:
- clean their hands.
- wear an apron.
- clean the NFAD hub or port cap (if present) with a disinfectant wipe and allow to air dry.
- check that the cannula is working by flushing the cannula with saline fluid before and after giving your IV bolus or infusion.
- check your cannula site and the dressing regularly and record and act on any changes
How you can take care of your cannula
- Keep your dressing clean and dry.
- You can usually wash, shower or bathe as normal but need to take care your cannula does not get wet, knocked or the connectors become open. If you are unsure or need help, please ask one of the clinical staff.
- Wear watches and other jewellery on the opposite hand or arm to avoid pulling on the cannula.
- Stay on the ward while your cannula is in use. For your safety, we ask that you do not leave the ward alone - especially if you are receiving fluids or medication, or if they are due soon. It is important that treatments are given at the right time and for the correct duration.
Please tell a member of staff if you have concerns about your cannula and immediately if:
- you feel pain, stinging, notice redness, bleeding, fluid leakage or swelling of or around the cannula site.
- you feel hot, cold or shivery.
- your fluid bag or bottle is empty.
- your cannula has an injection cap (port) and it is open.
- your cannula dressing is wet, dirty or loose.
- your cannula has dislodged (moved) or comes out.
- your cannula is difficult or painful to flush.
When will the cannula be removed?
A trained member of staff will remove your cannula:
- when your procedure or treatment has finished.
- if the cannula is not being used and there is no future plan to use it.
- if the skin around the insertion site is red or sore.
- if the cannula has dislodged or is leaking.
- before you are discharged from hospital (unless IV treatment is planned to continue at home).
Once removed, please observe the cannula site for the next
24 hours for any signs of redness, pain or leakage. If any of these are noticed, please contact the healthcare team looking after you.
Contact information
If you have any questions about the procedure, your treatment or cannula care, please ask a member of the healthcare team looking after you. You may be given further information or contact details from the area where you are being treated.