Update on National Patient Safety Alert: Philips CPAP and BiPAP devices
In August 2021, a National Patient Safety Alert was issued by the Medicines and Healthcare products Regulatory Agency (MHRA) for some Philips CPAP and CPAP-like devices.
We have been running a large-scale programme over the last 3 years to contact patients and many devices have now been changed.
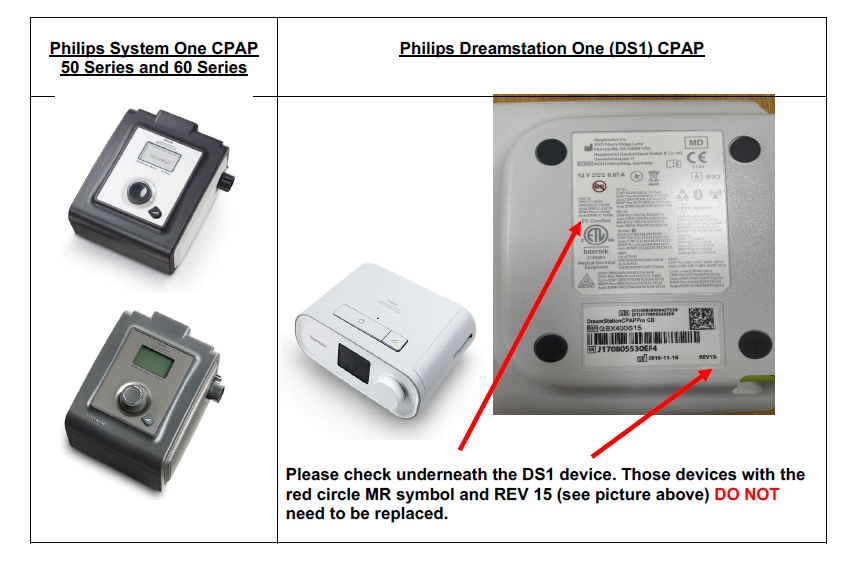
The affected devices we are replacing are CPAP System One and Dreamstation 1. There are dreamstation 1 devices out there which will have been repaired or issued from new without the problem (see picture below).
National Patient Safety Alert
The alert is in response that under certain conditions, Philips CPAP and CPAP-like devices, the foam part of the machine can be damaged.
These conditions include:
- very high temperatures;
- high humidity;
- use of a non-approved cleaning solution;
The MHRA is advising that reports of incidents related to this issue are rare and no incidents of harm have been reported in the UK.
We want to reassure patients that the risk of the device being effected are very low and you should continue to use the device.
Full information is available on the MHRA website.
Contacting us
We now have CPAP machines available for replacing affected devices. If you have not already been contacted or have not had your machine replaced please do not hesitate to contact us to arrange replacement.
Please contact us on 0300 422 6819 between 9-4 Monday to Friday or you can email us at ghn-tr.lung.function@nhs.net. You may be asked to leave a voicemail message.
Please bear with us, we are receiving a high number of calls and at the moment. If you leave a message, we will get back to you as soon as we can.
Is your device affected by this issue?
If your device is not a Philips device, it will not be affected by this issue. If you have a Philips device, please check the list of devices affected by the field safety notice on the Philips website.
Using your device
You should continue to use your device. Stopping treatment at this stage is likely to outweigh potential risks of using the devices.
The MHRA has advised that patients should continue to use these devices.
Can I arrange an appointment to discuss any concerns?
Yes. Our team can be contacted on 0300 422 6819 between 9-4 Monday to Friday or you can email us at ghn-tr.lung.function@nhs.net. The team can discuss any concerns and give you advice relevant to your situation.